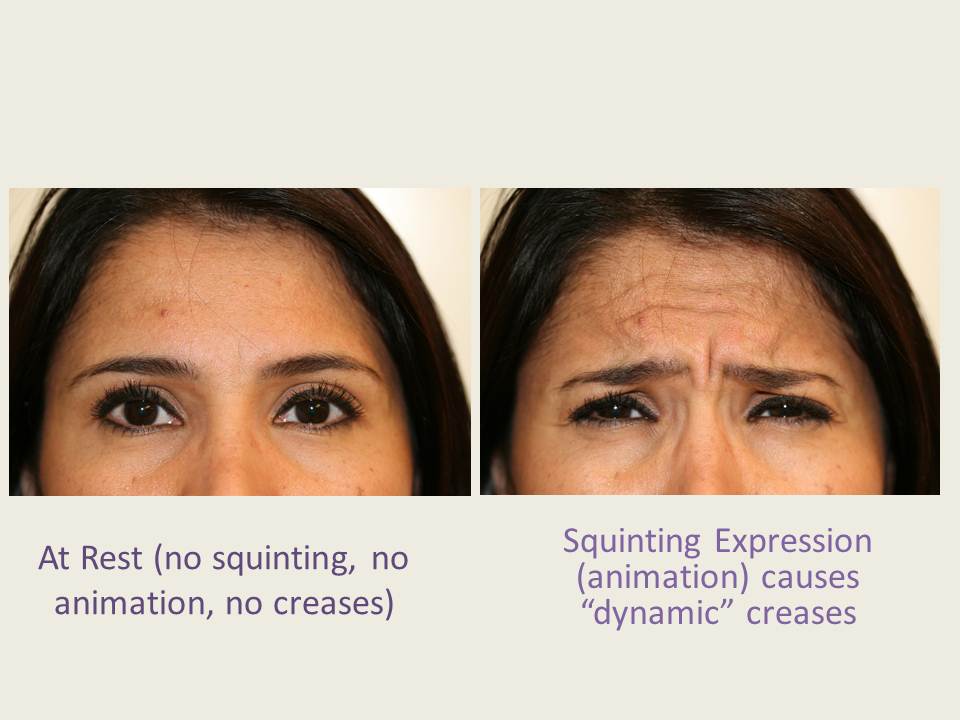
Neuromodulators we use at Feel Beautiful are FDA approved injectable products that causes “worry lines” and other dynamic creases to literally disappear from your face. Dynamic creases are wrinkles or folds of the skin that are not present when your face is relaxed. Only with facial expression do these lines appear. The folds of skin cause others to believe you are worried, anxious, mad, or concerned.
Interestingly, you can still express these emotions after neuromodulator treatment, but perhaps not so angrily. By relaxing facial muscles and preventing these lines of anger and worry, Botox ® Cosmetic treatment prevents them from deepening and becoming “static” creases. Regular neuromodulator treatment in the office prevents permanent lines in your facial skin from ever developing! Your face will appear more youthful for many years than it would be without neuromodulator treatment. Botox ® Cosmetic was the first FDA approved neuromodulator introduced into the United States market, but the effect is equivalent to other FDA approved neuromodulators Dysport® from Valeant Pharmaceuticals and Xeomin® from Merz Aesthetics. Dr. Laverson is among the most experienced physicians with Botox Cosmetic® in San Diego, having been a “gold” injector for over a decade and the first to introduce Botox Cosmetic® treatment on a large commercial scale. Most commonly treated lines and creases include the “squint” or “anger” lines between eyebrows, the horizontal “surprise” lines across the forehead, and the “crow’s feet” skin lines at the sides of the eyes.
Botox Cosmetic Injections in San Diego
Whether you’re new to the world of cosmetic injectables or they’re already an important part of your self-care and beauty routine, you’ve undoubtedly heard of BOTOX Cosmetic®. According to the 2019 Plastic Surgery Statistics Report, BOTOX® and other formulas containing Botulinum Toxin Type A were the most popular cosmetic minimally invasive treatments by far. With 7.7 million procedures performed, they surpassed soft-tissue fillers by 5 million! As one of the most effective treatments for temporarily minimizing the appearance of facial wrinkles, BOTOX Cosmetic® has become a household name for one main reason: because it works.
How does BOTOX Cosmetic® work?
BOTOX Cosmetic® contains Botulinum Toxin Type A, which is produced by a bacterium that helps to eliminate dynamic facial wrinkles by relaxing the muscles that cause them. Dynamic wrinkles are caused by facial expressions such as squinting and frowning, worry and anger. BOTOX Cosmetic® treatments prevent the muscle contractions, resulting in a more relaxed, youthful appearance that lasts for months.
It’s important to note that BOTOX Cosmetic® does not work on static wrinkles caused by sun damage, gravity, and chronological aging. Those wrinkles are usually best treated with dermal fillers, and many of our clients choose to combine the two for optimal results.
BOTOX Cosmetic® can be used as a wrinkle treatment to smooth:
- Glabellar lines – “the 11s” between the brows
- Horizontal forehead lines
- Periorbital lines – crow’s feet branching out from the outer corners of the eyes
- Bunny lines – on the bridge of the nose
- Perioral lines – above the upper lip
- Neck bands
- Chin dimpling
What you can expect from your BOTOX Cosmetic® treatment in a Medical Spa
Prior to your first San Diego BOTOX Cosmetic® treatment at the Feel Beautiful medical spa, Dr. Laverson will perform a thorough assessment of your areas of concern and design a fully customized treatment plan to help you achieve your desired results. During the treatment, you’ll be made as comfortable as possible before Dr. Laverson carefully and strategically injects the BOTOX Cosmetic® formula into your targeted muscles.
After your BOTOX treatment in San Diego, you can go back to your daily routine right away. Any redness and swelling from BOTOX injections should subside within an hour or two. You may have some minor bruising the following day, but it can be easily covered with makeup, if necessary.
Most patients begin to see results from their BOTOX Cosmetic® treatment within 3 to 5 days, though it may take up to a week to see the full BOTOX effects. Typically, the BOTOX effect and results last 3 to 4 months.
Will my facial expressions continue to look natural after Botox injections?
When it comes to BOTOX Cosmetic® and other injectables, precision and artistry are key to achieving the most beautiful results while maintaining the natural expressions that make you look like you. It’s important to work with an experienced practitioner who can assess the size of your muscles and accurately calculate how much Botox you will need. Frozen, expressionless faces are usually the result of inexperienced injectors overestimating the amount of BOTOX® needed.
Dr. Laverson understands facial anatomy and aesthetic features of forehead beauty, and he will incorporate his professional knowledge, experience, and judgment into your BOTOX treatment, in order to help you eliminate wrinkles (wrinkle relaxers), and eye rejuvenation, while still preserving facial expression and a youthful, natural appearance.
How many BOTOX units in a syringe?
Each BOTOX® vial contains 100 units of the injectables BOTOX® formula which must be reconstituted (mixed) with saline (sterile salt water) in order to adjust the strength and to achieve desired effects. Your syringe is filled with the exact number of BOTOX® units for your treatment plan.
How many BOTOX units per area?
Every BOTOX treatment plan in San Diego is different, but the following should give you a general idea of the number of BOTOX® units for each treatment area:
- Forehead BOTOX ® – 10 to 20 units
- Glabellar lines BOTOX ® – 5 to 15 units
- Crow’s feet BOTOX ® – 5 units (per side)
- Upper lip area BOTOX ® – 2 to 5 units
- Chin BOTOX ® – 2 to 10 units
During your consultation, Dr. Laverson will be able to give you a clearer picture of how many BOTOX® units he expects to use for your particular treatment plan.
What are the risks for BOTOX® treatment?
BOTOX® side effects are rare and temporary. They may include slight bruising, headaches, and in only 1% of cases, unintended temporary localized muscle weakness.
How much does BOTOX cost in San Diego?
The cost of BOTOX Cosmetic® treatments varies depending on the size of the treatment area, the depth of the wrinkles treated, and the desired results. All of these factors will determine the amount of BOTOX® required. At Feel Beautiful in San Diego, we can give you an estimate of the cost of your BOTOX Cosmetic® treatment during your consultation, after we’ve had a chance to evaluate your treatment area. But typically, BOTOX Cosmetic® treatments at Feel Beautiful range between $100 and $300.
If you’re looking to combat the signs of aging and rejuvenate your appearance with BOTOX®, you’ll be thrilled with your results in the hands of Dr. Laverson. His patients repeatedly appreciate his great results, and a great treatment experience. You will, too! Call today for your FREE consultation, (858) 295-4001
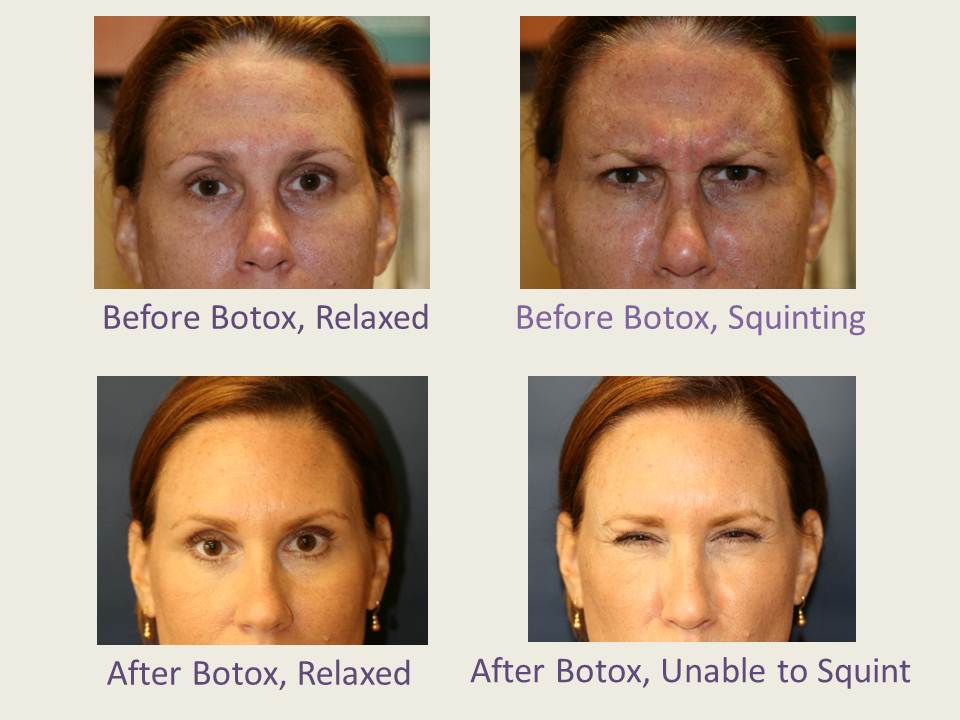
Botox Before & After
BOTOX® Cosmetic (onabotulinumtoxinA) Important Information
IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
Please see BOTOX® Cosmetic full Prescribing Information including Boxed Warning and Medication Guide
BOTOX® Cosmetic is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procures muscle activity in patients 18 to 65 years of age.
Postmarketing reports indicate that the effects of BOTOX® Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, blurred vision, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life-threatening, and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity, but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have underlying conditions that would predispose them to these symptoms. In unapproved uses, including spasticity in children and adults, and in approved indications, cases of spread of effect have occurred at doses comparable to those used to treat cervical dystonia and at lower doses.
BOTOX® Cosmetic is contraindicated in the presence of infection at the proposed injection site(s) and in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation.
The recommended dosage and frequency of administration for BOTOX® Cosmetic should not be exceeded. Risks resulting from administration at higher dosages are not known.
The potency Units of BOTOX® Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, Units of biological activity of BOTOX® Cosmetic cannot be compared to or converted into Units of any other botulinum toxin products assessed with any other specific assay method.
Please refer to Boxed Warning for Distant Spread of Toxin Effect.
No definitive, serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX® Cosmetic at the labeled dose of 20 Units (for glabellar lines) have been reported.
Injections In or Near Vulnerable Anatomic Structures
Care should be taken when injecting in or near vulnerable anatomic structures. Serious adverse events including fatal outcomes have been reported in patients who had received BOTOX injected directly into salivary glands, the oro-lingual-pharyngeal region, esophagus and stomach. Some patients had pre-existing dysphagia or significant debility. (Safety and effectiveness have not been established for indications pertaining to these injection sites.) Pneumothorax associated with injection procedure has been reported following the administration of BOTOX near the thorax. Caution is warranted when injecting in proximity to the lung, particularly the apices.
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, urticaria, soft-tissue edema, and dyspnea. If such reactions occur, further injection of BOTOX® Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent and, consequently, the causal agent cannot be reliably determined.
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junctional disorders (eg, myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of BOTOX® Cosmetic.
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
Caution should be used when BOTOX® Cosmetic treatment is used in patients who have an inflammatory skin problem at the injection site, marked facial asymmetry, ptosis, excessive dermatochalasis, deep dermal scarring, thick sebaceous skin, or the inability to substantially lessen glabellar lines by physically spreading them apart.
Patients should be counseled that if loss of strength, muscle weakness, or impaired vision occur, they should avoid driving a car or engaging in other potentially hazardous activities.
Co-administration of BOTOX® Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (eg, curare-like nondepolarizing blockers, lincosamides, polymyxins, quinidine, magnesium sulfate, anticholinesterases, succinylcholine chloride) should only be performed with caution as the effect of the toxin may be potentiated.
The effect of administering different botulinum neurotoxin serotypes at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
Administration of BOTOX® Cosmetic is not recommended during pregnancy. There are no adequate and well-controlled studies of BOTOX® Cosmetic in pregnant women.
It is not known whether BOTOX® Cosmetic is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when BOTOX® Cosmetic is administered to a nursing woman.
The most serious adverse events reported after treatment with botulinum toxin include spontaneous reports of death, sometimes associated with anaphylaxis, dysphagia, pneumonia, and/or other significant debility.
There have also been reports of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease.
The most frequently reported adverse events following injection of BOTOX® Cosmetic include blepharoptosis and nausea.
Contact Us
Address:
Phone:
Business Hours:
Mon-Fri
9:00 AM to 5:00 PM

 Book Now
Book Now